The FDA has issued a recall for certain eye drops after receiving a consumer complaint.
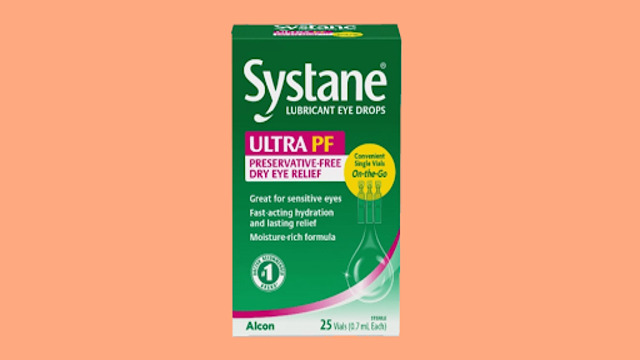
Systane Lubricant Eye Drops have been voluntarily recalled following a discovery of possible fungal contamination, the U.S. Food and Drug Administration (FDA) announced. The recall affects one batch of the product, specifically the Systane Lubricant Eye Drops Ultra PF, which were sold in single-use vials. The decision to recall the product was prompted by a consumer complaint about foreign material found inside one of the sealed vials.
The FDA's investigation revealed that the foreign material in question was fungal in nature, leading to the decision to pull the affected lot from the market. This fungal contamination presents a serious risk to users, especially those with weakened immune systems. If the contaminated drops are used, they could potentially cause eye infections that might result in vision loss or, in very rare cases, even be life-threatening for individuals with compromised immune systems.
Alcon Laboratories, the company responsible for manufacturing the Systane eye drops, has emphasized that there have been no reports of adverse effects or illnesses linked to the product thus far. The company reassured the public that the contamination appeared to be isolated to the single vial returned by a customer, and the recall was issued as a precautionary measure to prioritize the safety of consumers.
The affected batch of Systane eye drops comes in a 25-count package of on-the-go single-use vials. These vials have a lot number of 10101 and an expiration date of September 2025. The product was sold primarily at Publix grocery stores, which has issued a notice about the recall.
Systane eye drops are commonly used to treat dryness and irritation in the eyes, providing relief from the burning and discomfort that often accompanies dry eye symptoms. They are typically sold in small, portable vials for convenience, especially for people who need to manage their dry eye condition while on the go.
Consumers who have purchased the recalled product are urged to stop using it immediately. They can return the product to the place of purchase for a refund or replacement. The FDA also advises that anyone who has used the recalled eye drops and is experiencing symptoms, such as eye discomfort or irritation, should seek medical attention from a healthcare provider promptly.
Alcon, in response to the recall, assured that it is conducting an ongoing investigation to understand the root cause of the contamination. However, they stated that the foreign material seems to be limited to the single unit that was returned. As a result, the company took the necessary step to recall the affected batch out of an abundance of caution, ensuring that the safety and well-being of consumers remain a top priority.
The recall of Systane eye drops highlights the importance of vigilance when it comes to product safety, particularly for items that come in direct contact with sensitive areas like the eyes. While the recall seems to be isolated to a specific lot, it serves as a reminder to consumers to always check product labels and lot numbers, especially in the case of medical products.