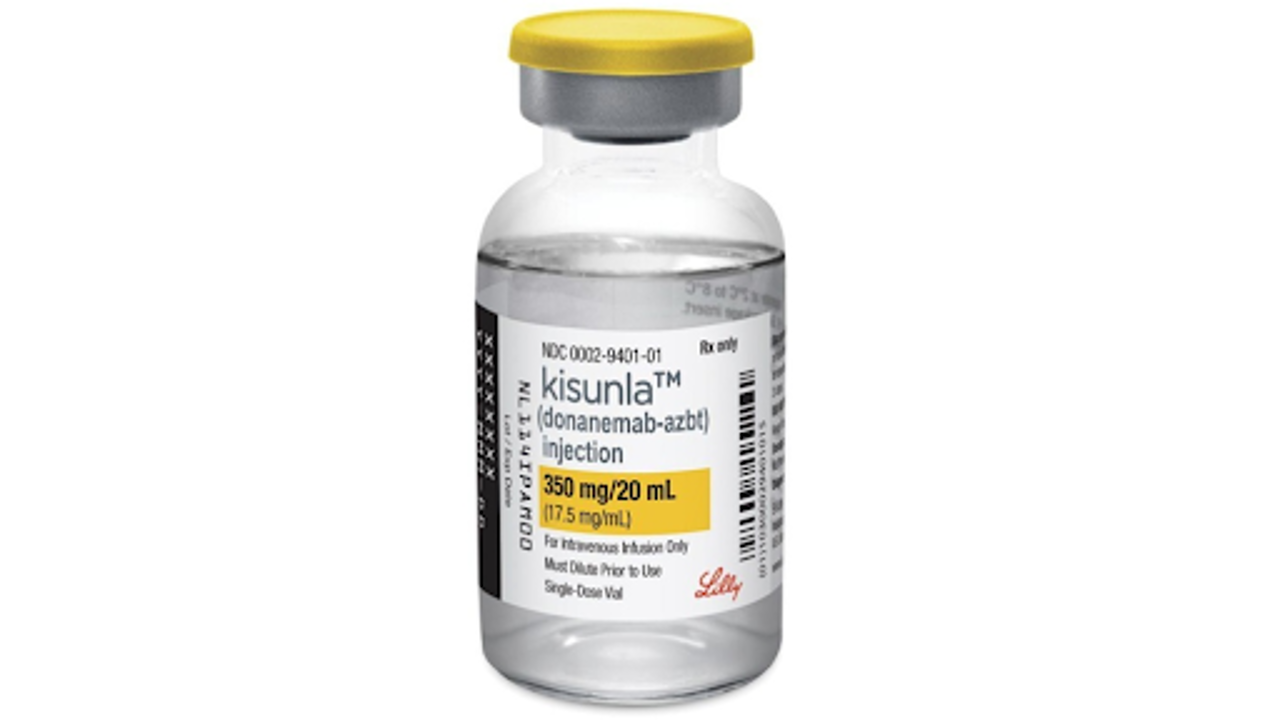
On Tuesday, July 2, 2024, the FDA gave the green light to Eli Lilly’s Kisunla for treating mild or early stages of Alzheimer’s-related dementia.
U.S. regulators have given the green light to a new Alzheimer’s drug, Kisunla by Eli Lilly, offering hope to those in the early stages of this devastating disease. This approval marks only the second drug proven to moderately slow cognitive decline in Alzheimer’s patients, following a similar drug approved from Japan last year.
The road to approval involved rigorous evaluation by the FDA, with Kisunla specifically targeting mild or early cases of Alzheimer’s. It works by combating the buildup of sticky amyloid plaques in the brain, a hallmark of the disease. The drug’s effectiveness was demonstrated in an 18-month study where patients showed a 22% slower decline in memory and cognitive ability compared to those on a placebo.
Dr. Suzanne Schindler, a neurologist at Washington University in St. Louis, expressed relief at having additional treatment options. For years, she’s watched Alzheimer’s patients deteriorate without effective therapies. However, while Kisunla offers some benefits, it comes with challenges like regular IV infusions and potential risks such as brain swelling.
The approval process included input from FDA advisors who endorsed the drug’s benefits, despite concerns about study methodologies. Lilly’s approach included allowing patients to stop treatment once their brain plaques were sufficiently reduced, potentially lowering long-term costs and risks associated with the drug.
Cost is another consideration. Lilly estimates Kisunla therapy at approximately $32,000 per year, slightly higher than its competitor, Leqembi. Moreover, logistical issues such as limited insurance coverage and the need for specialized infusion centers could hinder widespread adoption of these new treatments.
Despite these challenges, caregivers and patients may find Kisunla’s once-a-month infusion schedule more manageable compared to Leqembi’s bi-monthly regimen. This flexibility could ease the burden on families and healthcare providers involved in Alzheimer’s care.
Looking ahead, healthcare providers will need to ensure patients are properly screened for eligibility and have access to appropriate facilities for treatment. This includes regular monitoring for potential side effects like brain swelling or bleeding, which are inherent risks with drugs targeting amyloid plaques.
Dr. Mark Mintun from Lilly’s neuroscience division emphasized the importance of healthcare providers being prepared to offer these therapies. The initial setup and ongoing maintenance of these treatments require specialized knowledge and resources, which may not be readily available in all healthcare settings.
In conclusion, while the approval of Kisunla represents a significant step forward in Alzheimer’s treatment, its real-world impact will depend on overcoming logistical, financial, and medical challenges. For now, it offers a glimmer of hope to patients and families affected by this debilitating disease, providing a new tool in the fight against Alzheimer’s.